Now starting my career in Big Pharma!
 GlaxoSmithKline logo (this image do not belong to me)
GlaxoSmithKline logo (this image do not belong to me)
Since August 2020 I joined GlaxoSmithKline in the Protein, Cellular, and Structure Sciences group as a Protein Biochemistry Investigator.
I am working on multiple projects in early search for new treatments in different diseases.
I am very happy for using my training at a high pace set to bring solutions to patients. Keeping in mind our values: Patient focus, integrity, respect for people and transparency.
I am working on multiple projects in early search for new treatments in different diseases.
I am very happy for using my training at a high pace set to bring solutions to patients. Keeping in mind our values: Patient focus, integrity, respect for people and transparency.
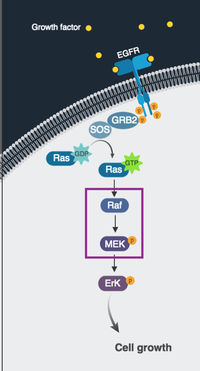
My Postdoc at University of Pennsylvania in Cancer Research!
My PhD at Cornell, I had two main projects working with Professor Linda Nicholson, doing NMR:
Rice project from the molecular aspects to the plant!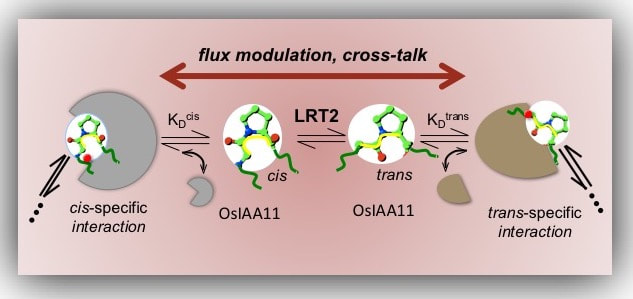 How the Prolyl Peptide Isomerases work, LRT2 is important in lateral root development in rice How the Prolyl Peptide Isomerases work, LRT2 is important in lateral root development in rice
The lateral root development in rice is known to be regulated by the plant hormone Auxin. In the presence of Auxin, the cis isomer of the transcription repressor protein OsIAA11 is degraded and lateral root development is initiated. Since the interconversion between cis and trans is a slow process, fast depletion of cis OsIAA11 isomer will create and unbalance accumulation of trans OsIAA11, thus, an enzyme that catalyze interconversion of trans to cis is necessary for appropriate signal regulation. Interestingly, deletion of an enzyme LRT2 in rice also affects lateral root initiation as does deletion of OsIAA11. In vitro, LRT2 was reported to cis/trans isomerize OsIAA11. For my PhD, I conducted NMR studies to quantify the interaction between LRT2 and OsIAA11. Through lineshape analysis, I found that LRT2 is optimally tuned for supplying maximum cis-OsIAA11 into the slower degradation pathway. Moreover, analysis of the data demonstrated that the catalyzed exchange rate (kex) is insensitive to the concentration of OsIAA11 at cellular levels, and scales linearly with the concentration of the enzyme. Also, we hypothesized that mutations in this enzyme could affect the isomerization rates of cis/trans and therefore affect the respond to lateral root initiation. I found mutants that reduce isomerization rates, the study of these mutants can provide important information on lateral root development. Rice is the third largest agriculture product worldwide and studying how this plant works could have a large environmental impact. Furthermore, this mechanism could be potentially generalized to all plants, and to any biological process affected by Auxin. These work is in the process of being published! We have published the NMR chemical assignments :link.springer.com/article/10.1007%2Fs12104-018-9803-x These studies were funded by the National Science Foundation award: MCB-1615350 and through the graduate student training grant support to me by the National Institutes of Health (2T32GM008267). |
Phosphorylation in EVH1 modified interaction with the proline rich region of Zyxin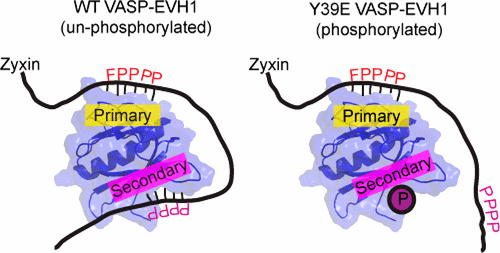 Image summarizing our finding, which is published in Biochemistry Journal Image summarizing our finding, which is published in Biochemistry Journal
The Vasodilator-Stimulated Phosphoprotein (VASP) regulates actin filament elongation, and thereby influences cell shape and migration. VASP localizes in the cell by its N-terminal Ena/VASP Homology (EVH1) domain, which binds to the consensus sequence motif (W/F)PxfP (tryptophan (W) or phenylalanine (F) followed by proline (P), any amino acid (x), an aliphatic amino acid (f), and proline). Many EVH1 binding partners contain multiple such motifs. One example is the cytoskeleton protein Zyxin, which is often concentrated on focal adhesions and along the actin cytoskeleton. Zyxin has four EVH1 binding motifs, suggesting that the consitutively tetrameric VASP might bind very tightly to Zyxin through avidity. A project I participated in from the beginning of my doctoral studies, which is part of my dissertation and resulted in a first-author publication, deals with this hypothesis. We found that the interaction between Zyxin and EVH1 is mainly a bivalent 1:1 Zyxin:EVH1 interaction, as opposed to the avidity effect that would create a 1:4 stoichiometry. This bivalent interaction is facilitated by a novel secondary binding site on the opposite face from the canonical binding site of EVH1, which we found by Nuclear Magnetic Resonance (NMR). We further showed that secondary site binding is inhibited by mutation of VASP-EVH1 residue Y39 to E, which mimics Abl-induced phosphorylation of Y39. Based on these findings, we proposed a model in which phosphorylation of Y39 acts as a stoichiometry switch that governs binding partner selection by the constitutive VASP tetramer. These results have broader implications for other multivalent VASP-EVH1 binding partners, and for furthering our understanding the role of Y39 phosphorylation in regulating VASP localization and cellular function. This work was published in Biochemistry: pubs.acs.org/doi/10.1021/acs.biochem.7b00618 this project was founded by the NSF award MCB-1157806 and the same training grant (2T32GM008267), as well as my dean's fellowship by Cornell. |
In previous years, I volunteer in Professor Robert Oswald’s group at Cornell. The research in this group focuses on the structure and dynamics of neurotransmitter receptors, specifically glutamate receptors. I gained experience in the expression and purification of the binding domain of glutamate receptors (iGluR2, iGluR3, iGluR6 and NR1) in order to run either nuclear magnetic resonance (NMR) spectroscopy, X-ray crystallography, isothermal titration calorimetry (ITC), or thermal denaturalization fluorescence experiments. I had the opportunity to practice all these techniques, except NMR, in this lab.